89 Homeostasis, Hypothermia, and Heatstroke
Homeostatis
In the previous unit we learned that the body is at best only 25 % efficient at converting chemical potential energy to useful work. The other 75% of the chemical potential energy becomes thermal energy, which the body exploits to manage body temperature as a part of homeostasis, or the body’s act of maintaining a relatively constant internal environment. Thermal injuries occur when body temperature becomes to high or too low, but our body has strategies for preventing that from occuring.

In order to analyze our body’s response to different environmental temperatures we need to first define temperature and clear up the definitions for some other thermodynamic quantities:
- Thermal energy(TE): Kinetic energy stored in the microscopic motion of atoms and molecules. The SI unit for thermal energy is Joules (J), though it is sometimes measured in calories or British Thermal Units (BTU)
- Temperature (T): A measure of the average thermal energy per atom or molecule. The SI unit for Temperature is Kelvin (K), though it is often measured in Celsius (°C) or degrees Fahrenheit (°F).
- Heat(Q):The amount of thermal energy transferred between an object and its environment due to a difference in the object and environment temperatures. The units for heat are the same as for thermal energy.
- Thermal Equilibrium: A state where the rate of heat transfer is zero because object and environmental temperatures are the same.
Reinforcement Exercise
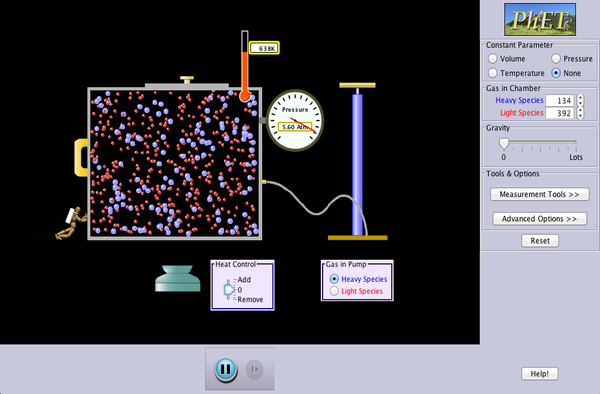
The following simulation allows you to visualize how temperature and atomic motion are related.
Thermal Injuries
Now that we have solid definitions for thermal energy, heat, temperature, and thermal equilibrium, we can follow the progression of thermal injuries:
- As a measure of the average motion of atoms and molecules, temperature influences the rate of chemical reactions and the ability of molecules, such as proteins, to remain in a particular shape.
- Due to the above, the body must maintain a relatively narrow range of temperature in order to function properly.
- The body is inefficient and thus converts chemical potential energy to primarily thermal energy as part of basic metabolism and when doing useful work.
- If heat transfer is limited, then thermal energy will build up in the body and temperature will increase, possibly resulting in hyperthermia and heat-related injuries such as heat stroke. We will learn to prevent hyperthermia by understanding how and when heat transfer to the environment is limited in the following chapters.
- If instead, heat is transferred to the environment faster than thermal energy can be converted from chemical potential energy, then body temperature falls, possibly leading to hypothermia and cold-related injuries such as frost-bite. We will learn to prevent hypothermia by understanding how and when heat transfer to the environment can become too fast in the following chapters.
- Human Body Temperature Scale by Foxtrot620 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons ↵
energy stored in the chemical bonds of a substance
work done on the external environment, such as moving objects, as apposed to work done internally, such as pumping blood
energy stored in the microscopic motion of atoms and molecules (microscopic kinetic energy)
a measure of the average kinetic energy of the particles (e.g., atoms and molecules) in an object, which determines how relatively hot or cold an object feels
is the state of steady internal physical and chemical conditions maintained by living systems
An amount of thermal energy transferred due to a difference in temperature.
a two systems are in thermal equilibrium when they do not exchange heat, which means they must be at the same temperature
The condition of having a body temperature well above the normal range.
The condition of having a body temperature well below the normal range.