84 Efficiency of the Human Body
The Energetic Functions of the Body[1]
All bodily functions, from thinking to lifting weights, require energy. The many small muscle actions accompanying all quiet activity, from sleeping to head scratching, ultimately become thermal energy, as do less visible muscle actions by the heart, lungs, and digestive tract. The rate at which the body uses food energy to sustain life and to do different activities is called the metabolic rate. The total energy conversion rate of a person at rest is called the basal metabolic rate (BMR) and is divided among various systems in the body, as shown the following table:
| Organ | Power consumed at rest (W) | Oxygen consumption (mL/min) | Percent of BMR |
| Liver & spleen | 23 | 67 | 27 |
| Brain | 16 | 47 | 19 |
| Skeletal muscle | 15 | 45 | 18 |
| Kidney | 9 | 26 | 10 |
| Heart | 6 | 17 | 7 |
| Other | 16 | 48 | 19 |
| Totals | 85 W | 250 mL/min | 100% |
The First Law of Thermodynamics
Heat
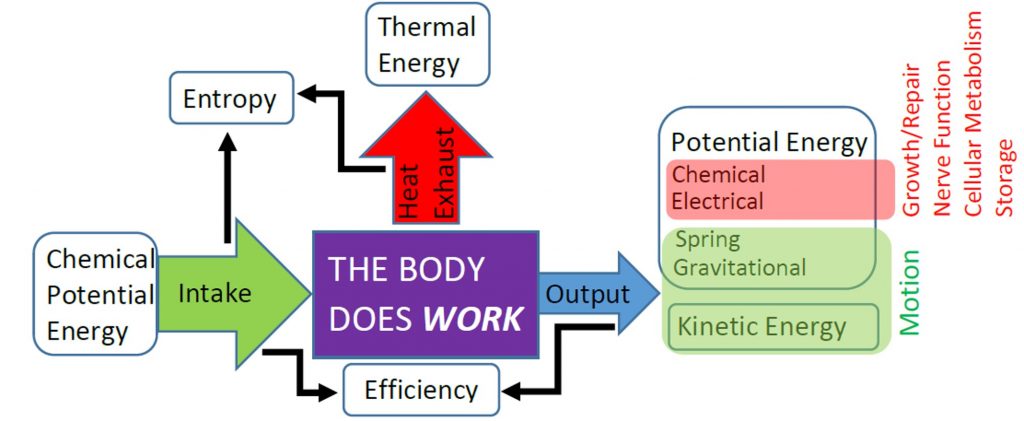
The body is capable of storing chemical potential energy and thermal energy internally. Remembering that thermal energy is just the kinetic energy of atoms and molecules, we recognize that these two types of energy are stored microscopically and internal to the body. Therefore, we often lump these two types of microscopic energy into the internal energy (![]() ). When on object is warmer then its surroundings then thermal energy will be transferred from object to surroundings, but if the object is cooler than its surroundings then thermal energy will transferred into the object from its surroundings. The amount of thermal energy exchanged due to temperature differences is often called heat (
). When on object is warmer then its surroundings then thermal energy will be transferred from object to surroundings, but if the object is cooler than its surroundings then thermal energy will transferred into the object from its surroundings. The amount of thermal energy exchanged due to temperature differences is often called heat (![]() ). When heat is transferred out of the body to the environment, we say call this exhaust heat, as indicated in the previous figure. We will learn more about how temperature and heat transfer are related in the next unit.
). When heat is transferred out of the body to the environment, we say call this exhaust heat, as indicated in the previous figure. We will learn more about how temperature and heat transfer are related in the next unit.
Energy Conservation
The Principle of Conservation of Energy states that energy cannot be created or destroyed. Therefore, if the body does useful work to transfer mechanical energy to its surroundings (![]() ), or transfer thermal energy to the environment as heat, then that energy must have come out of the body’s internal energy. We observe this throughout nature as the First Law of Thermodynamics:
), or transfer thermal energy to the environment as heat, then that energy must have come out of the body’s internal energy. We observe this throughout nature as the First Law of Thermodynamics:
(1) ![]()
Heat Engines
Your body uses chemical potential energy stored internally to do work, and that process also generates thermal energy, which you release as exhaust heat. The internal combustion engines that power most cars operate in similar fashion by converting chemical potential energy in fuel to thermal energy via combustion, then converting some of the thermal energy into useful work and dumping some into exhaust heat. Your body is capable of releasing the chemical potential energy in your food without combustion, which is good, because you are not capable of using thermal energy your from yourinternal energy to do work. Machines that can use thermal energy to do work, such as a combustion engine, are known as heat engines. Heat engines are still governed by the First Law of Thermodynamics, so any exhaust heat must have been thermal energy that was not used to do work. The thermal energy input that can be used to do work rather than wasted as exhaust heat determines the efficiency of the heat engine.
Body Efficiency
The efficiency of the human body in converting chemical potential energy into useful work is known as the mechanical efficiency of the body. We often calculate the body’s mechanical efficiency, as a percentage:
(2) ![]()
The mechanical efficiency of the body is limited because energy used for metabolic processes cannot be used to do useful work. Additional thermal energy generated during the chemical reactions that power muscle contractions along with friction in joints and other tissues reduces the efficiency of humans even further.[2].
Reinforcement Exercises
“Alas, our bodies are not 100 % efficient at converting food energy into mechanical output. But at about 25 % efficiency, we’re surprisingly good considering that most cars are around 20 %, and that an Iowa cornfield is only about 1.5 % efficient at converting incoming sunlight into chemical [potential energy] storage.” [3]For an excellent discussion of human mechanical efficiency and comparisons with other machines and fuel sources, see MPG of a Human by Tom Murphy, the source of the previous quote.
Everyday Example: Energy to Climb Stairs
Assuming a 20% mechanical efficiency in climbing stairs, how much does your internal energy decrease when a 65 kg person climbs a 15 m high flight of stairs? How much thermal energy does the person transfer to the environment as exhaust heat?
First, lets calculate the change in gravitational potential energy:
![]()
The person did work in converting chemical potential energy in their body to mechanical energy, specifically gravitational potential energy. However, they are only 20% efficient, which means that only 1/5 of the chemical potential energy they use goes into doing useful work. Therefore the change in chemical potential energy must have been 5x greater than the mechanical work output
![]()
The chemical potential energy used came out of the person’s internal energy so:
![]()
We can use the First Law of Thermodynamics to find the thermal energy exhausted by the person:
(3) ![]()
Rearranging for ![]() :
:
![]()
We find that the heat is negative, which makes sense because the person exhausts thermal energy out of the body and into to the environment while climbing the stairs.
Alternatively, we could have known right off that the exhaust heat must be 4/5 of the total loss of internal energy, because only 1/5 went into doing useful work. So the exhaust heat should be:
![]()
Food Calories
Everyday Examples
What fraction of a bagel would you need to eat in order to make up for the 47,775 J loss of internal energy (as chemical potential energy) that we calculated in the previous everyday example about climbing stairs?
There are 1,464,400 J/bagel
Therefore we need to eat:
![]()
Reinforcement Exercises
Conservation of Mass and of Energy
The digestive process is basically one of oxidizing food, so energy consumption is directly proportional to oxygen consumption. Therefore, we can determine the the actual energy consumed during different activities by measuring oxygen use. The following table shows the oxygen and corresponding energy consumption rates for various activities.
| Activity | Energy consumption in watts | Oxygen consumption in liters O2/min |
| Sleeping | 83 | 0.24 |
| Sitting at rest | 120 | 0.34 |
| Standing relaxed | 125 | 0.36 |
| Sitting in class | 210 | 0.60 |
| Walking (5 km/h) | 280 | 0.80 |
| Cycling (13–18 km/h) | 400 | 1.14 |
| Shivering | 425 | 1.21 |
| Playing tennis | 440 | 1.26 |
| Swimming breaststroke | 475 | 1.36 |
| Ice skating (14.5 km/h) | 545 | 1.56 |
| Climbing stairs (116/min) | 685 | 1.96 |
| Cycling (21 km/h) | 700 | 2.00 |
| Running cross-country | 740 | 2.12 |
| Playing basketball | 800 | 2.28 |
| Cycling, professional racer | 1855 | 5.30 |
| Sprinting | 2415 | 6.90 |
Everyday Examples: Climbing Stairs Again
In the previous examples we assumed stated that our mechanical efficiency when climbing stairs was 20%. Let’s use the data in the above table to verify that assumption. The data in the table is for a 76 kg person climbing 116 stairs per minute. Let’s calculate the rate at which that person did mechanical work while climbing stairs and compare that rate at which they used up internal energy (originally from food).
The minimum standard step height in the US is 6.0 inches[4] (0.15 m) then the gravitational potential energy of a 76 kg person will increase by 130 J with each step, as calculated below:
![]()
When climbing 116 stairs per minute the rate of energy use, or power, will then be:
![]()
According to our data table the body uses 685 W to climb stairs at this rate. Let’s calculate the efficiency:
![]()
As a percentage, this person is 32% mechanically efficient when climbing stairs. We may have underestimated in the previous examples when we assumed a 20% efficiency for stair climbing.
Reinforcement Exercises
We often talk about “burning” calories in order to lose weight, but what does that really mean scientifically?. First, we really we mean lose mass because that is the measure of how much stuff is in our bodies and weight depends on where you are (it’s different on the moon). Second, our bodies can’t just interchange mass and energy — they aren’t the same physical quantity and don’t even have the same units. So how do we actually lose mass by exercising? We don’t actually shed the atoms and molecules that make up body tissues like fat by “burning” them. Instead, we break down the fat molecules into smaller molecules and then break bonds within those molecules to release chemical potential energy, which we eventually convert to work and exhaust heat. The atoms and smaller molecules that resulting from breaking the bonds combine to form carbon dioxide and water vapor (CO2 and H2O) and we breath them out. We also excrete a bit as H2O in sweat and urine. The process is similar to burning wood in campfire — in the end you have much less mass of ash than you did original wood. Where did the rest of the mass go? Into the air as CO2 and H2O. The same is true for the fuel burned by your car. For more on this concept see the first video below. The really amazing fact is that your body completes this chemical process without the excessive temperatures associated with burning wood or fuel, which would damage your tissues. The body’s trick is to use enzymes, which are highly specialized molecules that act as catalysts to improve the speed and efficiency of chemical reactions, as described and animated in the beginning of the second video below.
General Efficiency
Similar to the body efficiency, the efficiency of any energetic process can be described as the amount of energy converted from the input form to the desired form divided by the original input amount. The following chart outlines the efficiencies of various systems at converting energy various forms. The chart does not account for the cost, hazard risk, or environmental impact associated with the required fuel, construction, maintenance, and by-products of each system.
| System | Input Energy Form | Desired Output Form | Max Efficiency |
| Human Body | Chemical Potential | Mechanical | 25 % |
| Automobile Engine | Chemical Potential | Mechanical | 25 % |
| Coal/Oil/Gas Fired Stream Turbine Power Plants | Chemical Potential | Electrical | 47% |
| Combined Cycle Gas Power Plants | Chemical Potential | Electrical | 58 % |
| Biomass/Biogas | Kinetic | Electrical | 40% |
| Nuclear | Kinetic | Electrical | 36% |
| Solar-Photovoltaic Power Plant | Sunlight (Electromagnetic) | Electrical | 15% |
| Solar-Thermal Power Plant | Sunlight (Electromagnetic) | Electrical | 23% |
| Hydroelectric and Tidal Power Plants | Gravitational Potential | Electrical | 90%+ |
Check out the energy systems tab in this simulation to visualize different energy conversion systems
- Significant content in this chapter was adapted from: OpenStax, College Physics. OpenStax CNX. Aug 30, 2019 http://cnx.org/contents/031da8d3-b525-429c-80cf-6c8ed997733a@17.22 ↵
- "The maximum work and mechanical efficiency of human muscles, and their most economical speed" by A. V. Hill,National Library of Medicine, U.S. National institutes of Health ↵
- "MPG of a Human" by Tom Murphy, Do The Math, UCSD Physics Department ↵
- "1917.120 - Fixed stairways." by Occupational Safety and Health Administration, United States Department of Labor ↵
- "Managing Water in the West: Hydroelectric Power" by Bureau of Reclimation, U.S. Department of the Interior ↵
- "Efficiency in Electricity Generation" by EURELECTRIC “Preservation of Resources” Working Group’s “Upstream” Sub-Group in collaboration with VGB, Union of the Electricity Industry ↵
- "Efficiency of Energy Conversion Devices" by John E. Dutton e-Education Institute, Penn State College of Earth and Mineral Sciences ↵
energy stored in the chemical bonds of a substance
the total of a systems thermal energy and chemical potential energy, the total energy stored microscopically within the system
energy stored in the microscopic motion of atoms and molecules (microscopic kinetic energy)
An amount of thermal energy transferred due to a difference in temperature.
heat transferred to the environment rather than being used to do useful work
Energy cannot be created or destroyed, only transferred from one type to another and/or from one object to another
the sum of potential and kinetic energy
the change in internal energy of a system is equal to the heat added to the system minus the work done by the system
A quantity representing the effect of applying a force to an object or system while it moves some distance.
the process of burning, the rapid chemical combination of a substance with oxygen, involving the production of heat and light
work done on the external environment, such as moving objects, as apposed to work done internally, such as pumping blood
devices for converting thermal energy to useful work and exhaust heat
ratio of useful work performed to total energy expended
the effectiveness of a machine in transforming the energy input to the device to mechanical energy output
a force that acts on surfaces in opposition to sliding motion between the surfaces
potential energy stored in objects based on their relative position within a gravitational field
unit of energy equivalent to 4.184 Joules
International standard (SI) unit of Energy
a measurement of the amount of matter in an object made by determining its resistance to changes in motion (inertial mass) or the force of gravity applied to it by another known mass from a known distance (gravitational mass). The gravitational mass and an inertial mass appear equal.
A quantity representing the capacity of an object or system to do work.